is the abbott test a naat test
Both give rapid results. ID NOW is an FDA approved CLIA-waived instrument which means.

Performance Of Point Of Care Tests For The Detection Of Chlamydia Trachomatis Infections A Systematic Review And Meta Analysis Eclinicalmedicine
The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.

. The methods below are acceptable for travelers between USA to Japan. Abbott IDNow is a rapid nucleic acid test for COVID-19 SARSCOV-2 which has a turnaround time of less than 1 hour. Im wondering if the airline agent are familiar with it.
A Nucleic Acid Amplification Test or NAAT is a type of viral diagnostic test for SARS-CoV-2 the virus that causes COVID-19. It is used on our ID NOW platform. Thus this is the key difference between NAAT and PCR.
Our negative covid test results reports are all different. The tests are available on our ARCHITECT and Alinity i systems. They bought out the Binax corporations test which was a rapid antigen test and began rebranding it as IDNow which was their umbrella term for relatively rapid infectious disease testing.
The term NAAT applies to a range of different technologies where nucleic acid ie RNA or DNA from a pathogen is amplified and detected to determine if a pathogen is present. NAATs detect genetic material nucleic acids. Abbott markets 2 different tests one is a NAAT and the other is a rapid antigen test.
Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. Abbott ID NOW Rapid Molecular Test NAAT This test can be used as clearance to return to work or to detect the virus in patients with exposure or symptoms. Categories Practice Test Tags ela cat test how many questions how many questions can you miss on the ncct test is the abbott test a naat test ncct practice test quizlet 2021 2022 All Practice Tests Printable Built with GeneratePress.
Order a NAAT Test Fill out and submit this form to order an Abbott ID NOW Rapid Molecular Test NAAT. Fast Shipping To USA Canada and. Abbott IDNow has a sensitivity which is less than standard PCR tests The above comes from a test centre in the US.
The former is a NAAT test the latter an antigen test. 8 Typically if the pathogen is a virus with an RNA genome like SARS-CoV-2 and influenza the first step in the NAAT method will be to convert the RNA into DNA using a reverse transcriptase and. Reply Report inappropriate content 6 replies to this topic taylorn Midland Texas Level Contributor 557 posts 107 reviews 52 helpful votes 1.
What COVID-19 Tests Does Japan Accept. IDNow and BinaxNOW are two different tests both Abbott products. NAAT is a molecular biological technique that amplifies genetic material using several ways such as polymerase chain reaction strand displacement or transcription-mediated amplification.
My husband is the only one that mention PCR NAAT my 15 years olds negative report says RT PCR covid 19 and mine said Lumiradx Sars-Cov-2. Brand and Generic products for sale. Is offering the Abbott ID Now COVID-19 Assay performed on the ID NOW Instrument.
Results 1 hour. Just go straight for a traditional PCR test to avoid any problems. I would say that this was a no-no.
Our accounting billing reporting and eligibility systems were designed and created specifically to support the unique administrative demands of a Consumer-Driven Health Plan. This test is a rapid molecular in vitro diagnostic test utilizing an isothermal nucleic acid amplification technology intended for the qualitative detection of nucleic acid from the SARS-CoV-2 viral RNA in direct nasal swabs. At Physicians Immediate Care we are able to offer the Abbott ID NOW COVID-19 Rapid Molecular PCR test This is a Nucleic Acid Amplification Test NAAT with results in under 15 minutes.
BUT only the test purchased from emed includes the necessary video appointment that is needed for travel documentation. Abbott received emergency use authorization EUA from the US. I now have my negative test from this morning but I am still in the process of hunting down my PCR test results.
Has anyone flying recently on United used the Abbott ID Now covid test for entry into Tahiti. Its a NAAT test which apparently is a type of PCR test. NAATs for SARS-CoV-2 specifically identify the RNA ribonucleic acid sequences that comprise the genetic material of the virus.
Reply Report inappropriate content 6 replies to this topic 1-6 of 6 replies Sorted by 1 taylorn Midland Texas Level Contributor 557 posts. Suggested to wait 5 days after initial exposure. PCR is a method in molecular biology that amplifies genetic material using thermal cycling.
The Binax is the one you can purchase at many outlets for home testing. Results are available in less than 15 minutes making it the fastest rapid point of care molecular COVID-19 test. Vitality team understands the strict Japan requirements for international travel.
Has anyone flying recently on United used the Abbott ID Now covid test for entry into Tahiti. Im wondering if the airline agent are familiar with it. For more information on our ARCHITECT antibody test check out this article.
Top-rated meds for sale now Is Abbott Id Now Covid Test A Naat Test. As a licensed full-service Third Party Administrator we are committed to Is Abbott Id Now Covid Test A Naat Test maintaining excellence and flexibility in the developing CDH marketplace. Rapid nicking enzyme amplification reaction NEAR Nucleic Acid Amplification Test NAAT technology ID Now by Abbott.
Its a NAAT test which apparently is a type of PCR test. Walk-in or reserve your time online. Pricing The Abbot NATT Test is 99.
Indeed this is very stressful. NAATs for SARS-CoV-2 test specimens from either. COVID-19 Antigen Testing Antigen tests work by testing a nasal or throat swab for the presence of unique proteins called antigens that make up part of the virus that causes COVID-19.
Abbott also developed separate lab-based serology blood tests to detect IgM and IgG antibodies that identify if a person has been previously exposed to the virus that causes COVID.

Id Now Covid 19 Point Of Care Abbott
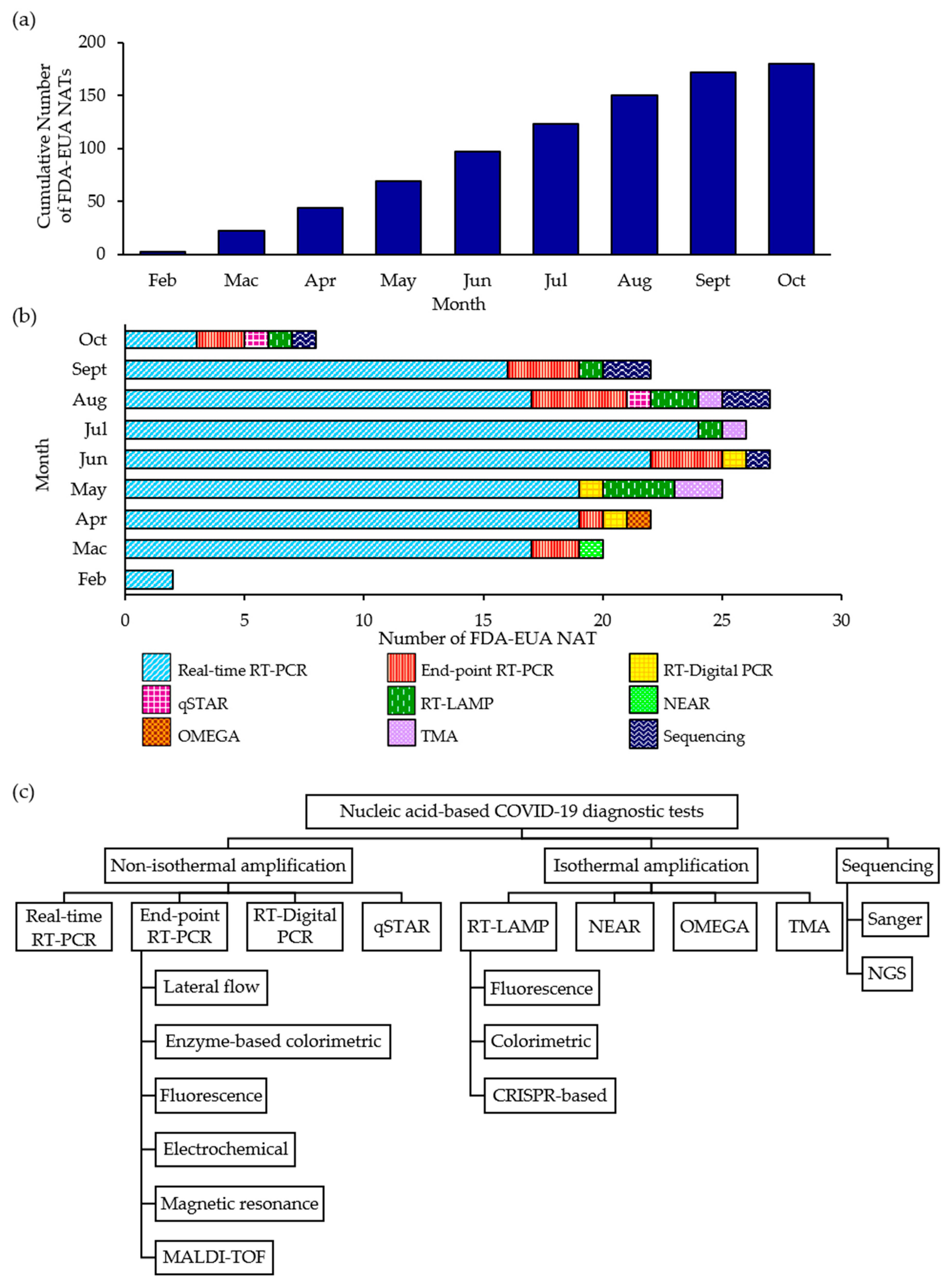
Diagnostics Free Full Text Nucleic Acid Based Diagnostic Tests For The Detection Sars Cov 2 An Update Html

Id Now Covid 19 Point Of Care Abbott

Use Of Covid 19 Assay On Abbott Id Now Instrument Interim Guidance Canada Ca
Id Now Covid 19 Point Of Care Abbott
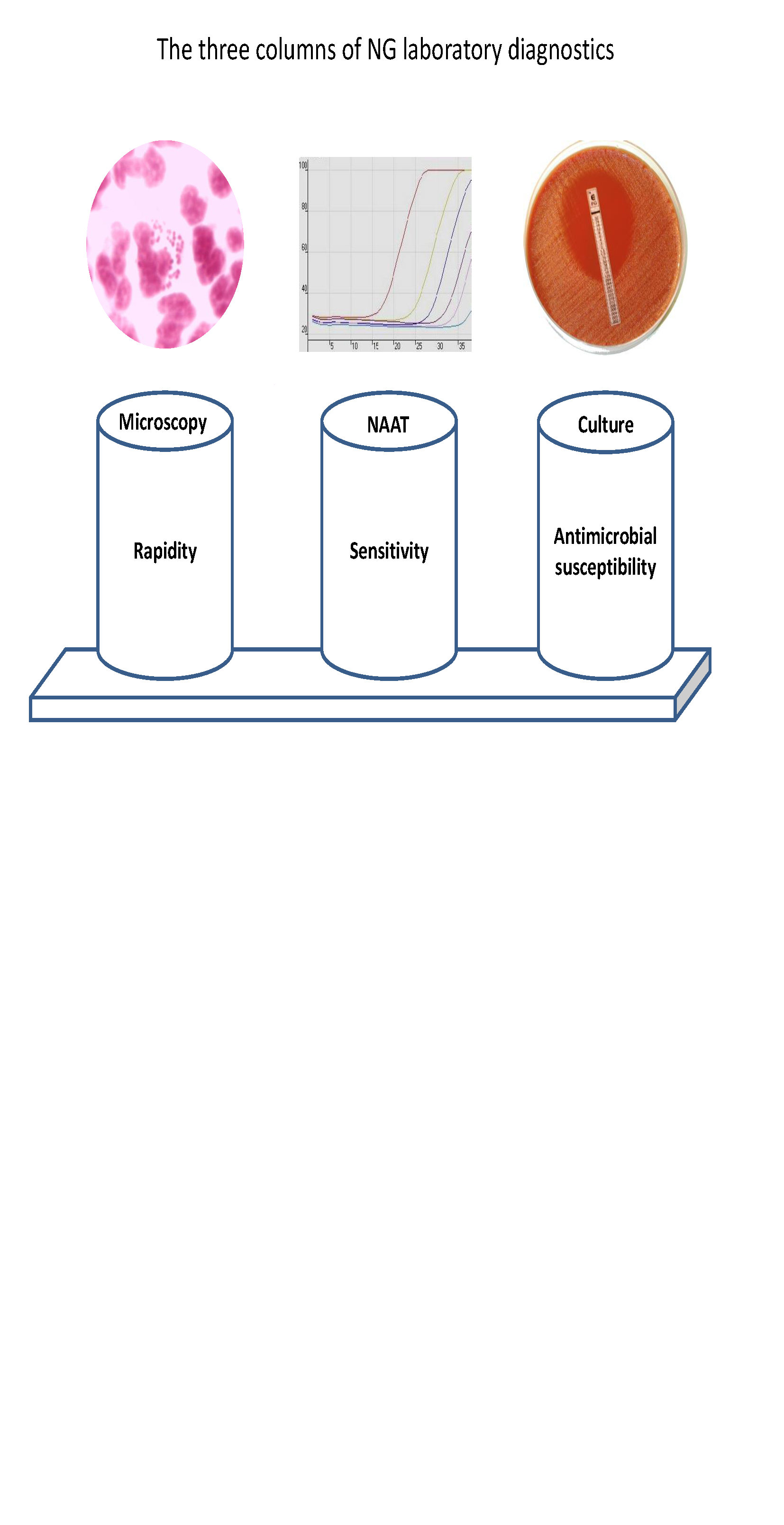
Pathogens Free Full Text The Laboratory Diagnosis Of Neisseria Gonorrhoeae Current Testing And Future Demands Html

Clinical Performance Of Id Now In Individuals With Compatible Sars Cov 2 Symptoms In Walk In Centres Accelerated Turnaround Time For Contact Tracing Ccdr 47 12 Canada Ca

Steps To Use Id Now Effectively Abbott Newsroom

Covid 19 Test Differences Antigen Vs Pcr Advanced Urgent Care

Id Now Eme 2021 Point Of Care Abbott

Id Now Covid 19 Point Of Care Abbott

Nucleic Acid Amplification Tests On Respiratory Samples For The Diagnosis Of Coronavirus Infections A Systematic Review And Meta Analysis Clinical Microbiology And Infection
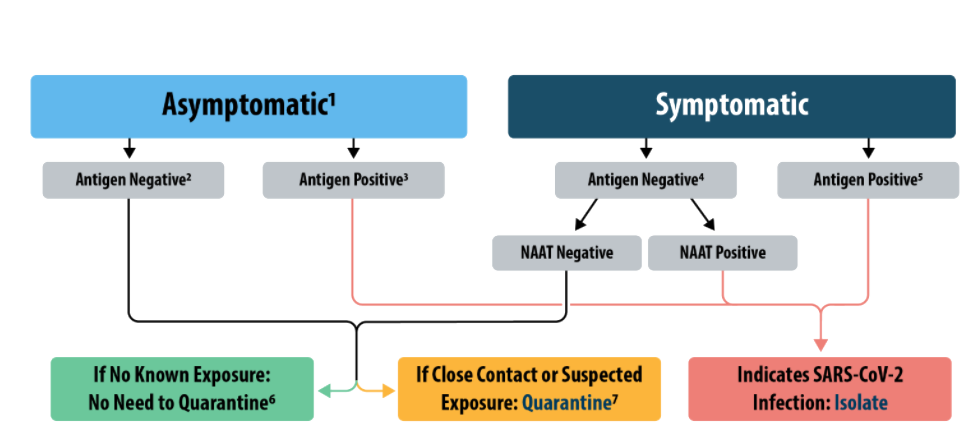
Covid 19 Testing Redicare Okemos

Covid 19 Testing Molokai Health Center

Id Now Express Test Nat Covitest Me

Rapid Home Tests For Covid 19 Issues With Availability And Access In The U S Issue Brief 9827 Kff

At Home Covid Tests Rapid Antigen Pcr Test Kits Walgreens

Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine
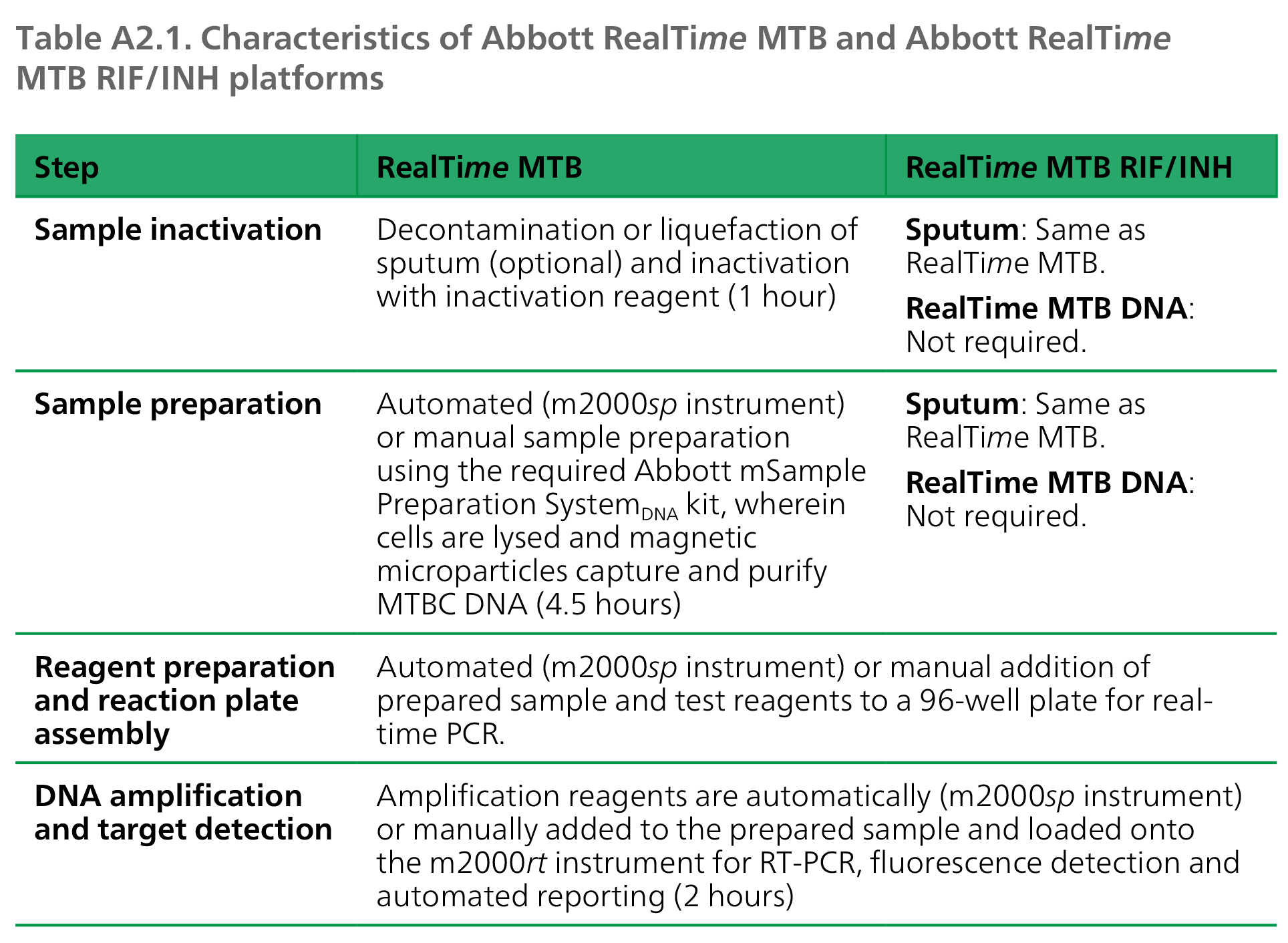
A2 1 Information Sheet Practical Considerations For Implementation Of The Abbott Realtime Mtb And Abbott Realtime Mtb Rif Inh Tests Tb Knowledge Sharing
Comments
Post a Comment